Sorting FAQs
Please note:
- Cell sorting is provided as a service, the FACSAriaII and FACSAria Fusion cell sorters are operated exclusively by the Flow Cytometry Facility staff.
- We have user rules, please read them and abide by them.
- All formal presentations or publications containing data from work performed at our Flow Cytometry Service Facility must be identified as such. The following statement is suggested: "We would like to acknowledge the support provided by the Flow Cytometry Facility of the Max Planck Institute for Molecular Genetics".
Co-authorship is suggested for extensive consultation on experimental design or data analysis.
Which cell sorter (FACS) should I book?
That, of course, strongly depends on your experiment. Please see the sorter’s configurations for further details.
First time users should request a project meeting before flow cytometry/cell sorting experiments. We recommend to discuss all new sort protocols in advance.
For detection of BFP, DAPI, Hoechst dyes or Brilliant UV (BUV)- and BrilliantViolet (BV) dyes, Pacific blue, Pacific Orange, V500, V450..., you can only use the FACSFusion, because it has a 355 nm and a 405 nm laser, the FACSAriaII doesn’t
Propidium iodide (PI), CFP, YFP, Venus, GFP, mCherry, dTomato, RFP, dsRed, PE, FITC, PerCP, APC, Alexa647, Cy5, Alexa633, Alexa488, APC-Cy7, Pe-Cy7 among others can be detected on both FACS machines (FACSAriaII and FACSFusion).
What do I need to bring to my sorting appointment?
Samples: pre-filtered through a 35 µm/70 µm/100 µm cell strainer (sorting samples and your controls. Cell strainer size must be smaller than that of the nozzle. Filtration of cells is mandatory.)
Vessel(s) for collecting the target cells with buffer (Cell culture media/PBS etc. No harmful volatile substances like 2-Mercaptoethanol or Trizol!). We recommend coating the inside walls of your collection vessels with your buffer of choice.
Which controls do I need?
We recommend the use of unlabeled cells, single stained cells, and FMO controls in addition to experimental controls for most (immune) phenotyping projects.
DNA cell cycle staining projects usually do not require unlabeled cells.
Questions regarding experimental design should be discussed with us prior to conducting the experiments.
What can I sort into?
You can collect your cells/particles into 2 mL or 1.5 mL Eppendorf tubes, 5 mL FACS tubes (4 populations simultaneously) OR 15 mL Falcon tubes (2 populations simultaneously) OR into one plate at a time (6/12/24/96/384 wells).
How many cells should I bring?
The number of cells to be prepared for flow cytometric experiments can vary greatly and is highly dependent on the detection assay and the purpose of the flow cytometric experiment.
It is ideal to start with an excess of cells; however, the size of the source may be the limiting factor. For cell analysis, it is always a good start to start with 1 x 10^6 cells, but less is also possible (sample volume should exceed 100 µL but please don’t dilute cells more than necessary.)
Analysis of rare events is only possible if large sample volumes (at an appropriate density) are acquired (plan booking time accordingly).
If your sample contains only a few thousand cells, we can analyze/sort them. In this case, please keep the sample volume between 100-200 µL.
What density should the cells be at?
Cells should be at approx. 10-20 million/ml. Please bring additional buffer to dilute the samples if needed. Note, as a rule of thumb, approx. 1 hour sorting time is required for 1 ml sample volume (with our 85 µm standard nozzle at flow rate 1).
How many populations can I collect at once?
Our sorters can sort into max. two 15 mL OR max. four micro tubes OR four 5 mL FACS round bottom tubes at one time. They are designed to collect only one population per well at a time for any of the plate configurations (6/12/24/96/384 wells).
What will be the volume of my sorted cell populations?
Drop volumes per sorted event, with our standard sort precision mask (essentially sorts only one drop per target event) are:
- 70 µm nozzle: ~1 nL (i.e. 1 million sorted cells result in ~1 ml volume on top of your collection medium in your collection vessel)
- 85 µm nozzle: ~2 nL (i.e. 1 million sorted cells equals approx.. 2 ml sorting volume)
- 100 µm nozzle: ~2.5 nL
- 130 µm nozzle: ~6 nL
How much time should I reserve?
We need sufficient time for setup, analysis and sorting of the samples as well as for clean-up. For orientation: SETUP: 15 minutes plus time to run compensation controls and setup template (10-15 min, can be longer in case of many comp. controls or low bead/cell numbers). SAMPLE: Rule of thumb: approx. 1 hour sorting time is required for 1 ml sample volume (lowest flow rate, 85 µm Nozzle, cell density: 10 million/mL). CLEANUP: 10 min.
Any nozzle change requires an additional 30 minutes of booking time.
Why should I stain dead cells?
Staining your sample for dead cells improves the quality of the sorted sample. When dead cells are marked and removed from the sorted population, the user obtains more viable cells for downstream experiments.
Dead cells tend to bind antibodies non-specifically, so you may analyze/sort false positives.
Dead cells may lose expression of a previously expressed fluorescent protein, allowing false-negative cells to be sorted.
What to do when your cells form aggregates?
We generally recommend to have a final concentration of 2-5 mM EDTA in your sample buffer. If your cells have a more pronounced tendency to form aggregates, you may consider one of the following modifications:
- use calcium/magnesium free buffer for your cells
- add EDTA (2 - 5 mM)
- add 25 µg/ml DNAse I + 5 mM MgCl2 (no EDTA then), if the cell preparation induces increased cell lysis
- add 1% Accutase in sorting buffer
Which sheath buffer is used in the cell sorters?
We use the Beckman Coulter Isoton II diluent. The ISOTON II diluent is a filtered, phosphate-buffered saline solution compatible with most human and rodent cell types, and may be used for the suspension of most biological cells.
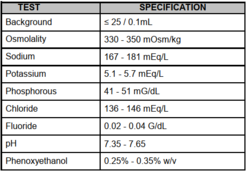
What is the maximum sort rate?
The maximum event rate for purification on the sorters should not surpass ¼ of the drop drive frequency. High speed sorting creates 60,000–90,000 droplets per second (70 mm nozzle). Low speed sorting creates 30,000–50,000 drops per second (85 µm nozzle, our standard). Special setups using atypical nozzles (100 µm, 130 µm) will deviate from these settings. In all cases, the cell concentration is adjusted to maintain sort efficiencies of 75-80% or usually better (>90%). We recommend diluent is brought to the appointment.
Is the sort sterile?
In one word: No. However, the sheath in our sorters is filtered through a 0.2 µm filter and the sample lines are regularly cleaned with BD FACS Clean, detergent and water, while surfaces are regularly liberally wiped with 70% ethanol. Our sorters are open to the air and are classed as clean and not sterile. Therefore if you want to culture your cells after sorting, we advise you to add pen/strep to your media. We also control for possible contamination by incubating the sorting stream sheath in media/LB/agar culture media at 37 °C. We have never had any problems with contamination.
How can I access my data?
We are saving your sort pdf (layout and sort report) and experiment (raw data) on our project drive “FlowCytometryLab”. You are responsible for saving your experiment files. No USB-keys or external harddrives allowed at the machine’s computers!
Windows users: you can download your files under: \\project\FlowCytometryLab\username (username is your MPI login name)
MAC users: please connect to: smb://project/FlowCytometryLabUser (take this literally!) Note: this is Mac specific and gets relayed to /project/FlowCytometryLab/username in the background
Linux users: /project/FlowCytometryLab/username (username is your MPI login name)
How do I acknowledge the Flow Cytometry Facility?
All formal presentations or publications containing data from work performed at our Flow Cytometry Service Facility must be identified as such. The following statement is suggested: "We would like to acknowledge the support provided by the Flow Cytometry Facility of the Max Planck Institute for Molecular Genetics".
Co-authorship is suggested for extensive consultation on experimental design or data analysis
Of course, anything more detailed, or acknowledgement of particular staff, is always welcome!
Thank you for your cooperation and help!
