Genetic diseases shift boundaries within the genome
Researchers in Berlin find that some rare diseases are caused by the destruction of functional boundaries within DNA
Recent investigations have shown that our genome and those of other mammals is partitioned into large functional units called topologically associated domains, or TADs for short. TADs are very long DNA sections containing one or more genes and their regulatory elements. An important function of TADs appears to be the formation of self-contained areas of gene regulation, and, at the same time, to isolate these from neighbouring areas. On the basis of three rare diseases in humans, scientists from the Max Planck Institute for Molecular Genetics and the Charité - Universitätsmedizin Berlin have now shown that shifts in the boundaries of TADs can lead to significant disruptions in the regulation of associated genes. TADs are therefore crucial for the proper functioning of genes. The researchers’ findings show that hereditary diseases can be caused not only by changes in coding genes themselves but also, surprisingly, by changes in non-coding regions located far from those genes.
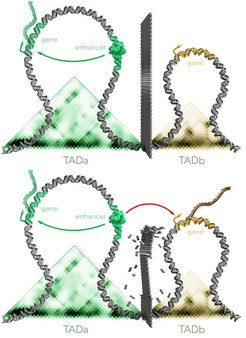
Illustration of two TADs in a healthy genome. Above: The presence of a “wall” between the two regions means that the regulator (= enhancer) in TADa can only influence the gene in TADa but not the gene in TADb.
Below: If the boundary between two TADs is altered or shifted due to a mutation, the regulator/enhancer can also influence genes that are normally shielded from it.
The human genome contains around 20,000 protein-coding genes. Surprisingly, the small roundworm C. elegans measuring just one millimetre in length has almost the same number of genes in spite of the fact that humans and roundworms differ radically in their biological complexity. This is because humans are able to better exploit their genetic potential, firstly by modifying gene products and secondly by using the same genes for a number of different functions.
This requires a high degree of regulation, as every cell in the body contains the same genetic information. Scientists estimate that around 40 percent of our genome is devoted to gene regulation. However, it remains unclear how it is ensured, which regulators influence – or do not influence – which genes.
TADs, topologically associated domains, play a key role in this respect: TADs are DNA sections, in which the DNA forms large three-dimensional structures consisting of histones, regulatory proteins and transcription factors. Each TAD comprises one or more genes together with all their regulatory elements. Their structure has been well conserved throughout evolutionary history and can be found in various cell types, as well as in various species. Regulatory elements within a TAD act only within “their” TAD; conversely, genes in neighbouring TADs are isolated from their influence. How the separation of one TAD from another is accomplished remains to be shown, but there is increasing evidence that so called boundary elements prevent the contact between TADs.
The importance of TADs in connection with the genesis of diseases has recently been investigated by scientists from the Max Planck Institute for Molecular Genetics and the Charité - Universitätsmedizin Berlin on the basis of three different hereditary diseases in humans. They discovered that changes, particularly at the boundaries of TADs, can lead to significant disturbances in gene regulation and cause associated diseases. The findings show that, because of this higher-level architecture of the genome, diseases can be caused not only by changes in coding genes themselves, but also by changes in non-coding regions located far from those genes.
In their investigations, the Berlin-based researchers focussed on three different rare human diseases that affect bones in the hands and feet. Brachydactyly is a hereditary shortening of the fingers and toes. In polydactyly, extra fingers or toes develop, and in syndactyly several fingers or toes fuse. All three disorders have genetic causes and arise during the course of embryonic development.
The researchers were able to show that the three diseases are due to different structural changes in the genome such as deletions, duplications, or inversions. In a deletion, a large section of DNA is missing, in a duplication, a DNA section is doubled, and in an inversion a large section of a DNA sequence is reversed. These changes disrupt the structure of TADs in the region concerned by shifting or removing their boundaries.
“Structural changes to the genome are a common cause of genetic diseases. The diagnostic standard for identifying these diseases therefore includes a search for such changes. However, the changes found are often difficult to interpret, and it then remains unclear whether they really are the underlying cause of the disease,” explains Stefan Mundlos, head of the Research Group at the MPIMP that is carrying out these investigations. The researchers therefore transferred the genetic changes found in human diseases to the genome of mice. To this end, they used a modification of the CRISPR/Cas technique previously developed in the group. It allows them to induce large structural changes in the murine genome within a short time. “Our results prove that these diseases in humans are caused by genomic changes: mice with the altered genome exhibit the same symptoms as affected humans.”
Until now, scientists had assumed that the loss of gene function must be caused by changes in the gene itself or in regulatory units of the genome, so called enhancers that control the activity of the gene. However, the results of the researchers in Berlin show that the environment of a gene can also have a strong influence on the proper function of a gene over a distance of several million bases.
“The DNA can be imagined as a long thread that is subdivided by walls into various sections or TADs,” explains Mundlos. “All elements within a section – genes, regulators, transcription factors, polymerases and many others – can interact freely. The walls separate the sections and shield them from neighbouring activity. However, individual walls may be removed or displaced by structural changes. This, in turn, alters the composition of the TAD, and suddenly, elements are able to interact that are normally strictly separated from each other. As a result, genes may be misregulated and, for example, cause the deformities we have been studying.” The findings of the Berlin-based researchers therefore shed new light on the mechanisms by which changes in the genome cause diseases.
The Research Group of Stefan Mundlos is receiving support from a private patron of the Max Planck Foundation within the framework of a project for modern methods of genome analysis for rare diseases.
PM/HR












