Regulation of euchromatin-heterochromatin crosstalk in stem cells
The early mouse embryo is characterized by the plasticity of cell fate choices, which depends on a flexible gene-expression program and a largely permissive chromatin environment. Embryonic stem cells and pluripotent cells of the blastocyst display a decondensed chromatin pattern with low levels of compact heterochromatin. These findings are in line with findings at the molecular level: relative to differentiated cells, ES cells have significantly constrained heterochromatin accumulation and lower levels of repressive histone modifications, such as H3K9me2/3, and higher levels of histone marks associated with transcribed regions, such as H3K4me3 or histone acetylation.
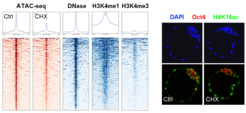
We have recently shown that the permissive chromatin state of embryonic stem cells is dependent on a high translational output and is acutely responsive to changes in protein synthesis, cellular growth and nutrient availability. However, an acute reduction in permissive chromatin does not immediately induce global heterochromatinization in pluripotent cells. How heterochromatin levels are controlled in pluripotent cells and whether euchromatin-heterochromatin crosstalk affects stem cell identity and potential remain unclear.
In this context, we are interested in identifying regulators of heterochromatin and euchromatin-heterochromatin crosstalk in embryonic stem cells. We combine latest gene editing methods with CRISPR screens to identify factors controlling stem cell chromatin.
Further reading
Bulut-Karslioglu et al., Cell Stem Cell 2018
