Mechanisms of embryonic diapause
Pluripotency provides the basis of embryonic development and regenerative medicine. Pluripotency is also remarkably dynamic, as it can alternate between different states (naïve, formative, primed) and can be promptly resolved to allow lineage specification. In nature, pluripotency only exists transiently. Under conditions conducive to proliferation, precise concentration and combination of morphogens promote pluripotency exit, thus cellular growth pathways, e.g. Fgf, Wnt, Bmp, Tgf, etc. govern differentiation. In stressful and unfavorable environments, development applies an emergency break in the form of sustaining the non-implanted embryo in a dormant state for longer periods to increase the chance of survival of the offspring. This phenomenon, called embryonic diapause is a reproductive strategy used by over 100 mammalian species.
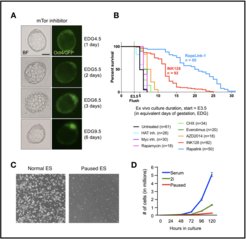
We have previously shown that the mTOR kinase, a major regulator of cell growth, governs embryonic diapause. Inhibition of mTOR (mTORi) pauses embryos at the blastocyst stage up to 30 days ex vivo (Panels A, B). mTORi-paused blastocysts retain regular morphology and the pluripotent stem cell pool as marked by Oct4 (Panel A). Upon release from mTORi and embryo retransfer, paused blastocysts can generate healthy, fertile offspring, indicating that ex vivo pausing does not compromise developmental potential. mTORi-paused embryos enter a dormant state with globally downregulated transcription and translation, and an altered metabolic profile. This state can be mimicked by continuous mTORi treatment of ES cells in culture (Panel C). Paused ES cells retain colony morphology and pluripotency markers, however exhibit nearly diminished cell proliferation (Panel D). Upon mTORi withdrawal, paused ES cells proliferate, retain ES markers, and contribute to high grade chimera, thus recapitulating the reversibility of mTORi-induced developmental pausing.
Further reading:
