The unique interior of nuclear condensates
New study by the Hnisz Lab in Nature
Human cells contain over 40 different types of proteinaceous droplets – known as biomolecular condensates. Condensates contain proteins and nucleic acids in unusually high amounts, and it has long been predicted that the environment inside condensates may be different than anywhere else in a cell. Testing this idea has so far been challenging for the lack of tools to change the inside of condensates in living cells. In a large collaborative effort Denes Hnisz’ lab has developed a unique tool for investigating the inside of condensates in living cells and has made some surprising discoveries.
Proteins in a cell are not arranged randomly, but are separated into small organelles. Organelles without a surrounding membrane are commonly referred to as biomolecular condensates. Many condensates and the proteins that are concentrated in them are involved in diverse cellular processes. Condensates are thought to have emergent properties, for example viscosity, that the individual molecules do not possess on their own. Until now, it has been difficult to investigate the relationships between the physical state of the biomolecules in condensates and their relationship to biological functions in cells.
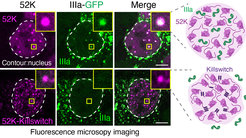
Yaotian Zhang and Henri Niskanen working together in Denes Hnisz’s lab discovered a short, 17-amino acid micropeptide they named the “Killswitch,” which they developed into a tool to selectively alter the physical state of biomolecules in condensates. They conjugated the killswitch to an anti-GFP nanobody and introduced it into cells containing various biomolecular condensates that were labeled with green fluorescent protein (GFP). The scientists observed unusual and surprising behavior of the biomolecules within the condensates, which in several instances dramatically affected their function:
They observed that Killswitch “glued” the GFP-labeled proteins within the condensates together, solidifying the otherwise dynamic, liquid-like droplets. The killswitch had the remarkable ability to solidify over a dozen different types of condensates in cells, including nucleoli, oncoprotein condensates, and viral condensates. Simply by restricting the mobility of one protein, the killswitch changed the inside of the condensates which inhibited the recruitment of other proteins into condensates.
Testing the effect of the killswitch on viral condensates, the researchers encountered another surprise. Viral proteins encoded by adenoviruses form a large condensate in which infectious viral particles are assembled. The researchers found that the killswitch was able to prevent the viral proteins from entering the condensates, so that no more infectious virus particles were produced.
Denes Hnisz explains the major surprise of the study: “The different viral proteins have the ability to interact in solution, but they cannot do that in a condensate once we target the condensate with the killswitch. This is one of the first experimental demonstration that the same protein behaves in a different way inside a condensate versus outside of it.”
The results of the study could be used for therapeutic purposes in the future. It is known that many different condensates occur in cancer cells. The scientists investigated cancer-specific condensates. “The data with our collaborators in Vienna showed, that we block cancer cell proliferation by solidifying condensates formed by nuclear oncoproteins with the killswitch”, said Yaotian Zhang, PhD student in the Hnisz lab, and first author on the study.
So far, the tool cannot yet be used to treat cancer. Further studies are needed to enable the micropeptide to bind specifically to the correct site of action in the cell. There are no universally applicable tools for manipulating all types of condensates in living human cells in a specific way.
“The tricky part is targeting the micropeptides specifically to the specific cells and specific locations within cells”, says Henri Niskanen, postdoctoral fellow, and corresponding author on the study.
The scientists therefore plan to develop further micropeptides that can recognize and bind every type of condensate in human cells. They also seek to test how these affect the function of the condensates.
The work was carried out by an interdisciplinary network of scientists. In addition to the laboratories of Tugce Aktas and Matt Kraushar, the MPIMG service groups FACS, microscopy, sequencing, and mass spectrometry were also involved. In addition, scientists from several international institutes were involved: the University of Veterinary Medicine Vienna, the St. Anna Children's Cancer Research Institute (CCRI), the Research Center for Molecular Medicine of the Austrian Academy of Sciences (CeMM) in Vienna, the Hospital of Philadelphia and the Perelman School of Medicine at the University of Pennsylvania in the US, and the University of Lausanne in Switzerland.












